Microscopy
Laboratory
Electron and Confocal Microscopy Laboratory
STRI offers both scanning electron microscopy and confocal microscopy services including processing of biological and non-biological specimens through fixation, dehydration, critical point drying and metallic coating.
| Scanning Electron Microscope | |
|---|---|
| STRI Staff, fellows, interns, volunteers | $15.00/hour |
| Panamanian researchers | $15.00/hour |
| Panamanian academic centers | $15.00/hour |
| Research associates and non STRI scientists | $25.00/hour |
| Comercial visitors and film crews | $100.00/hour |
*Private companies or NGO’s
| Scanning Laser Confocal Microscope | |
|---|---|
| STRI staff | $10.00/hour |
| Panamanian academic centers | $10.00/hour |
| STRI fellows, interns and volunteers | $15.00/hour |
| Panamanian researchers | $15.00/hour |
| Research associates and non STRI scientists | $30.00/hour |
| Comercial visitors and film crews | $100.00/hour |
*Private companies or NGO’s
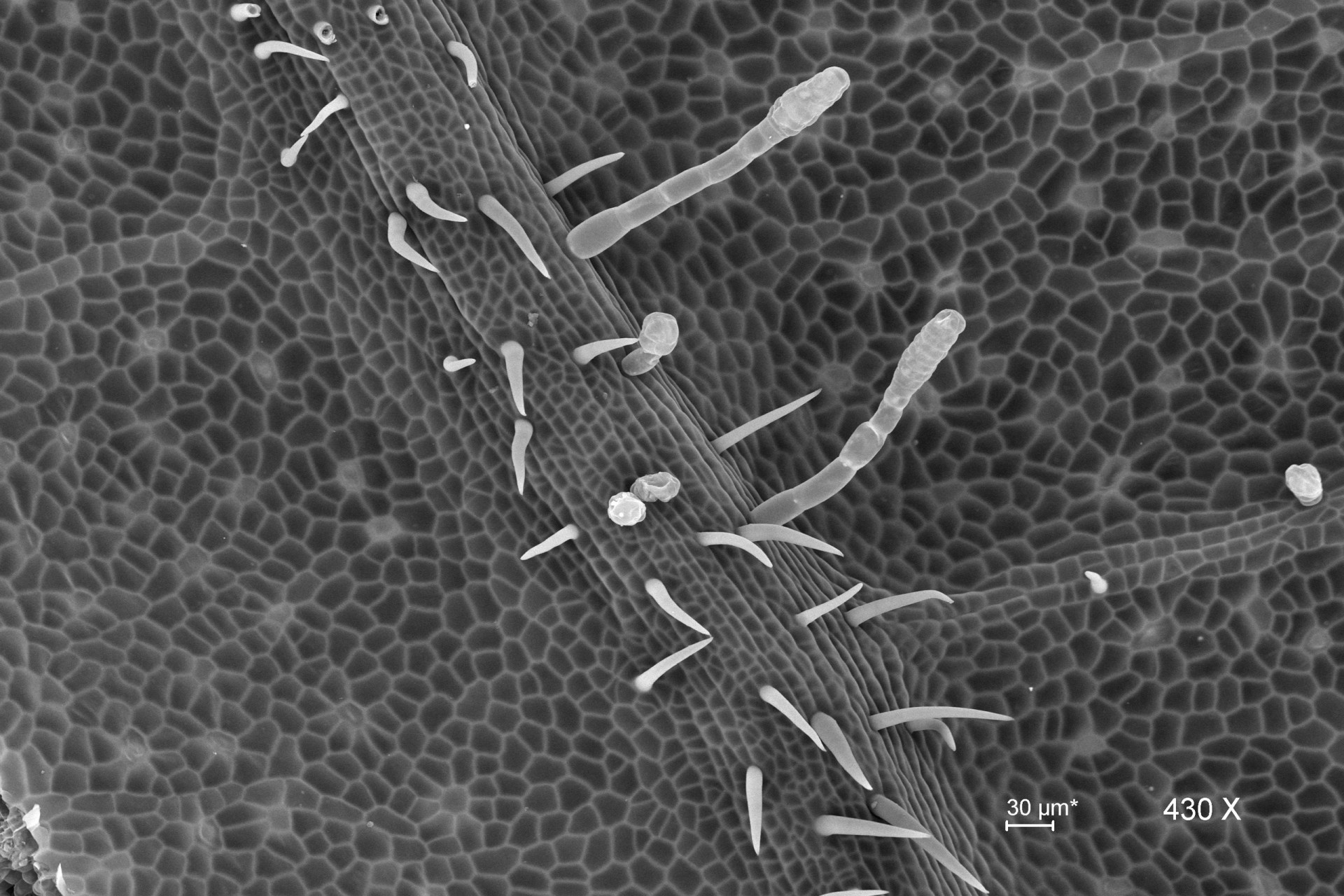
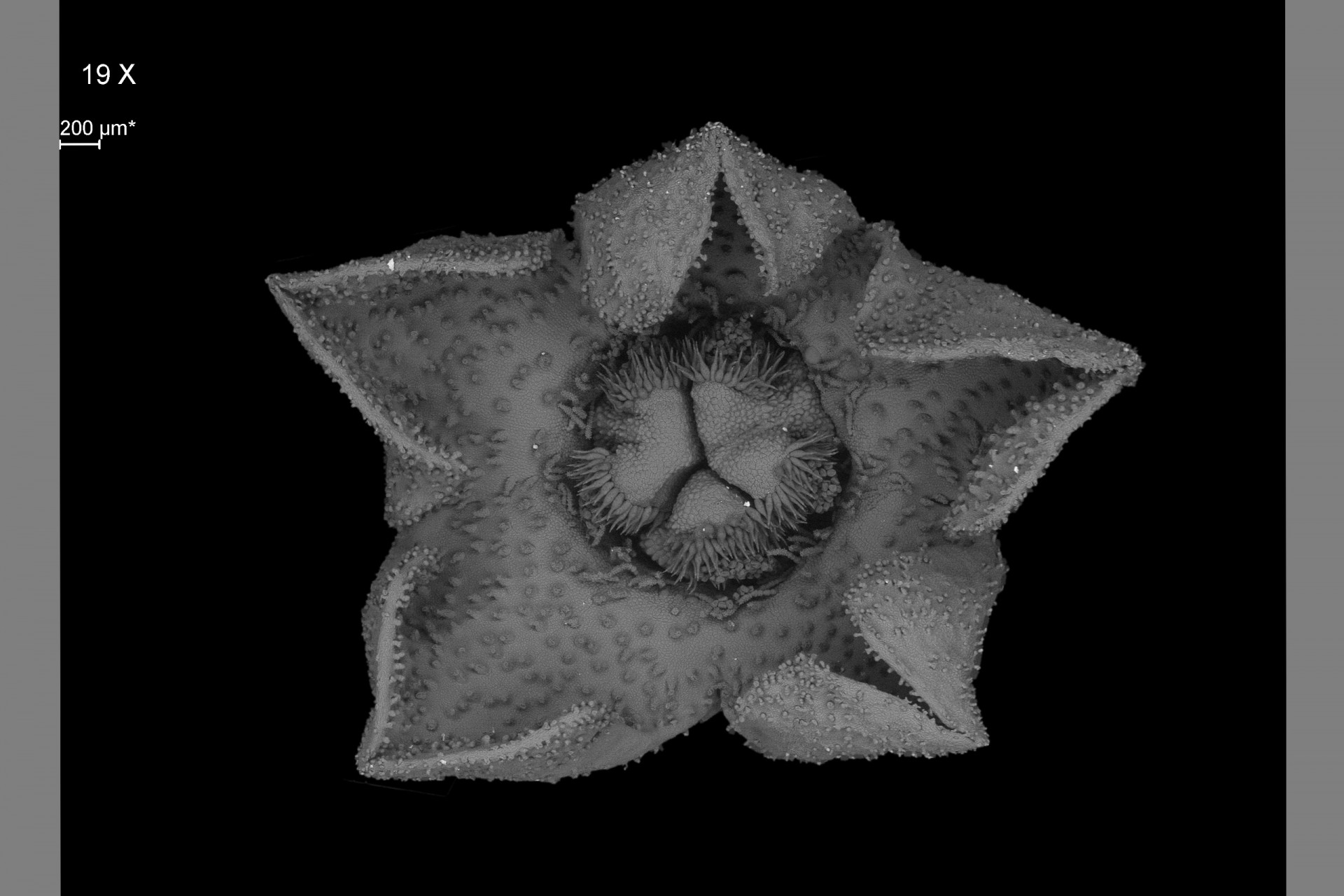
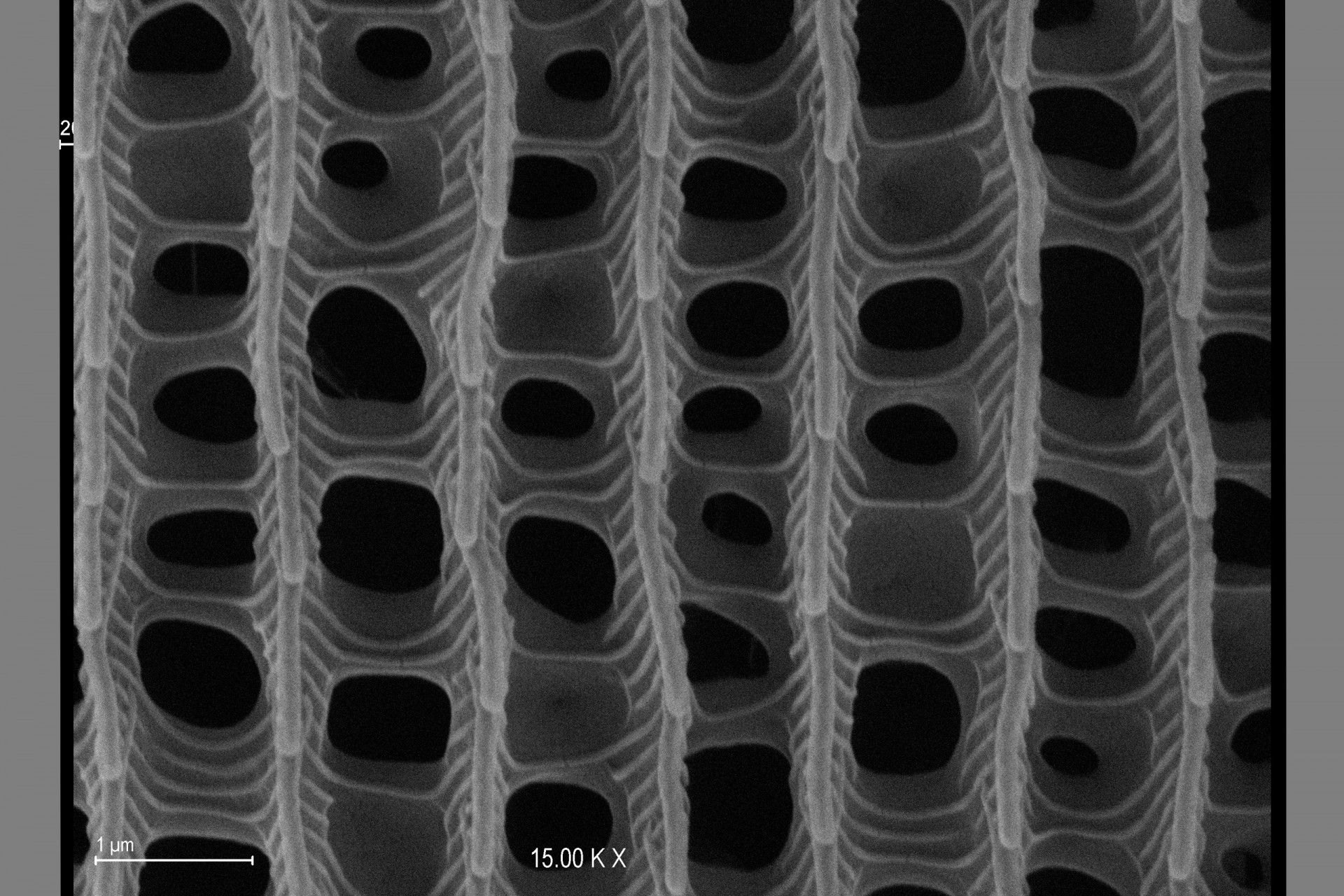
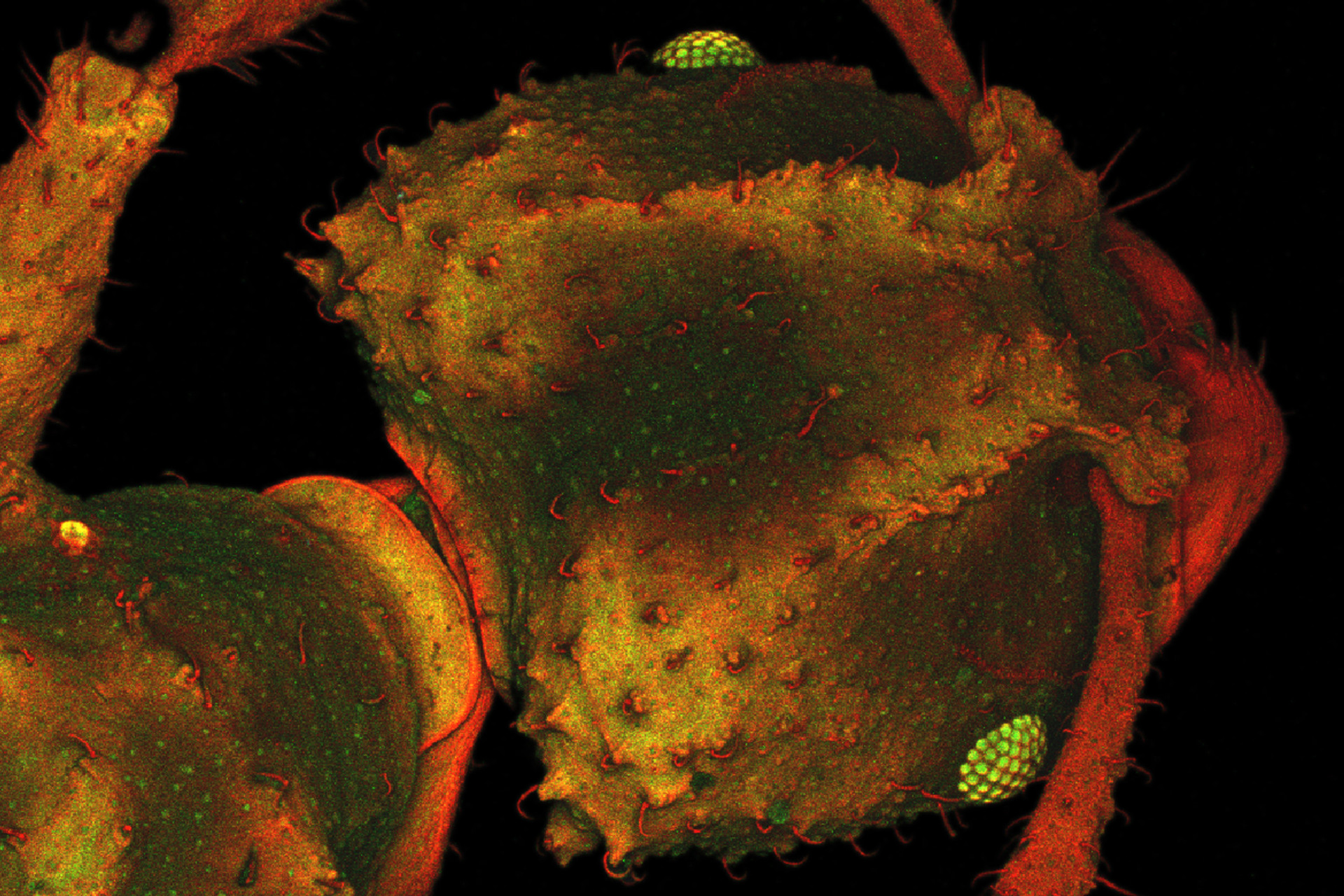
Scanning Electron Microscopy
Scanning electron microscopy (SEM) is a common technique used by researchers in natural and materials sciences. SEM takes advantage of the oscillation properties of electrons to magnify specimens up to a million times at a resolution of less than 10 nanometers.
The latest advances in SEM allow direct observation of biological materials without prior processing (fixation or dehydration), resulting in fewer structural alterations. In scanning mode, using transmitted electrons (STEM), specimens prepared on grids for transmission electron microscopy (TEM) can also be observed.
This facility is available to researchers and industries in fields the biological sciences (Botany, Entomology, Marine Biology, Paleontology), health, materials science and other related fields.
SEM/STEM Services include:
Surface observation and or cryofracture techniques.
Direct observation of fresh specimens via cold mounts at variable pressure (SEM).
Observation of histological cuts or particulate material via Scanning Transmission Electron Microscopy (STEM).
Equipment
Zeiss Evo 40 VP electronic scanning microscope includes secondary electron detector for observation in high vacuum and under variable pressure, backscattered electron detectors for observation under variable pressure and in transmitted electron observation mode (Scanning Transmission Electron Microscopy).
Details/Specifications
Detectors:
Secondary electron detector (SED).
Quad backscatter electron detector (QBSED).
Variable pressure secondary electron detector (VPSE).
Energy Dispersive Spectrometer (EDS) detector
Observations:
Observation of specimens in high vacuum with secondary and backscattered electron detector (ES/BSE).
Observation of specimens at variable pressure with secondary and backscattered electron detector (ES/BSE).
Observation of fresh biological material without the need of metallic coating or the use of fixers, thereby decreasing the risk of contamination and the possible presence of artifacts on the specimen.
Observation of ultrathin slices with electrons transmitted through the STEM system.
Use of cold mounts, permitting the observation of fresh specimens.
EDS is an ideal all-purpose qualitative and quantitative energy dispersive microanalysis system for bulk specimens, polished samples thin layers, particles and rough surfaces for industry, research and education.
Critical point drying using a Denton Vacuum DCP-1 critical point dryer (Critical Point Drying Apparatus C P D-1), very fragile specimens that could be deformed by oven or air drying, can be dried.
Metallic coating: An Anatech Hummer VI-A (Hummer VI-A Sputtering System) metallic coater is used on specimens that need to be observed in high vacuum. In this process, specimens are coated with a thin layer of gold/palladium (60%-40%) to make them conducive to the passage of secondary electrons.
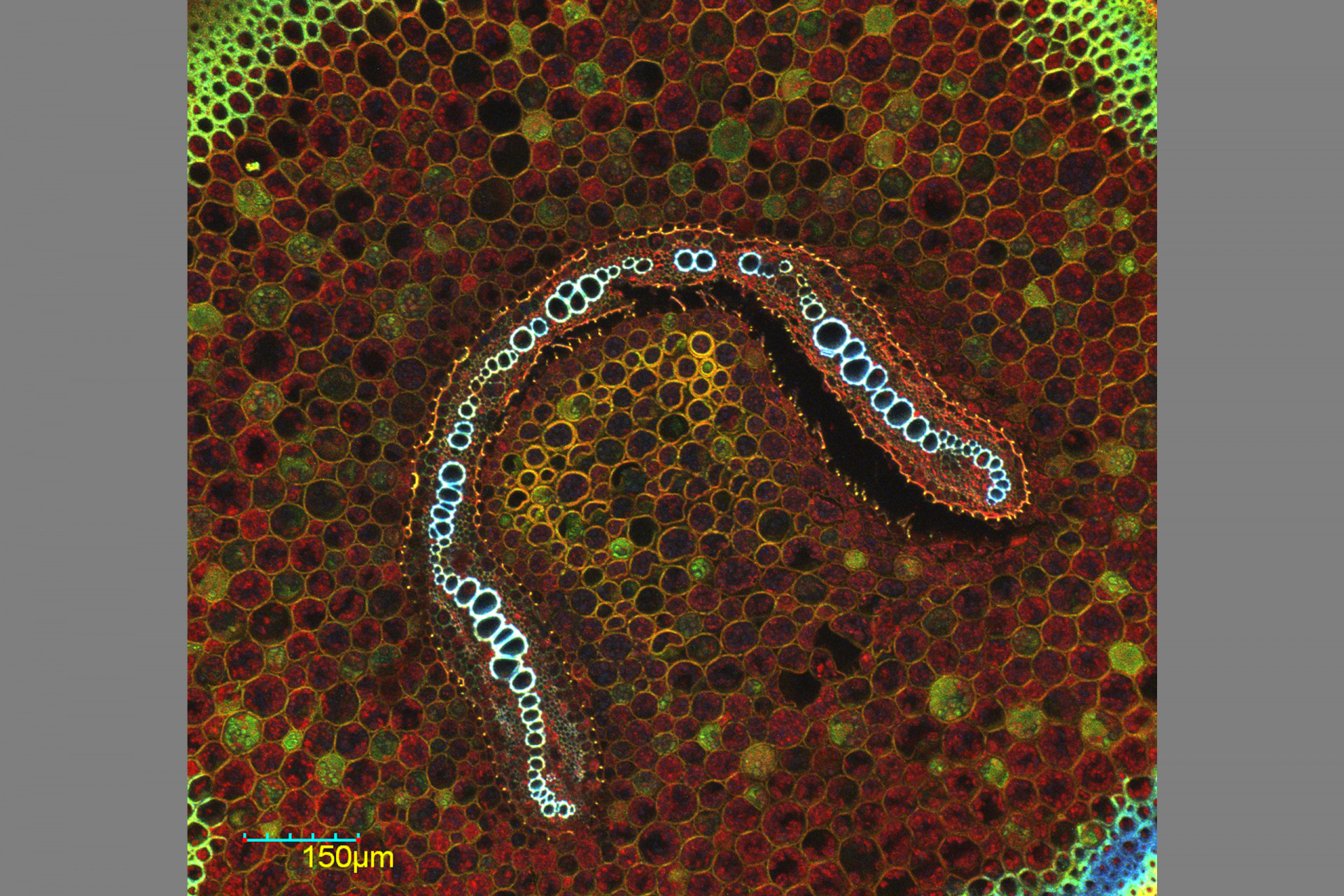
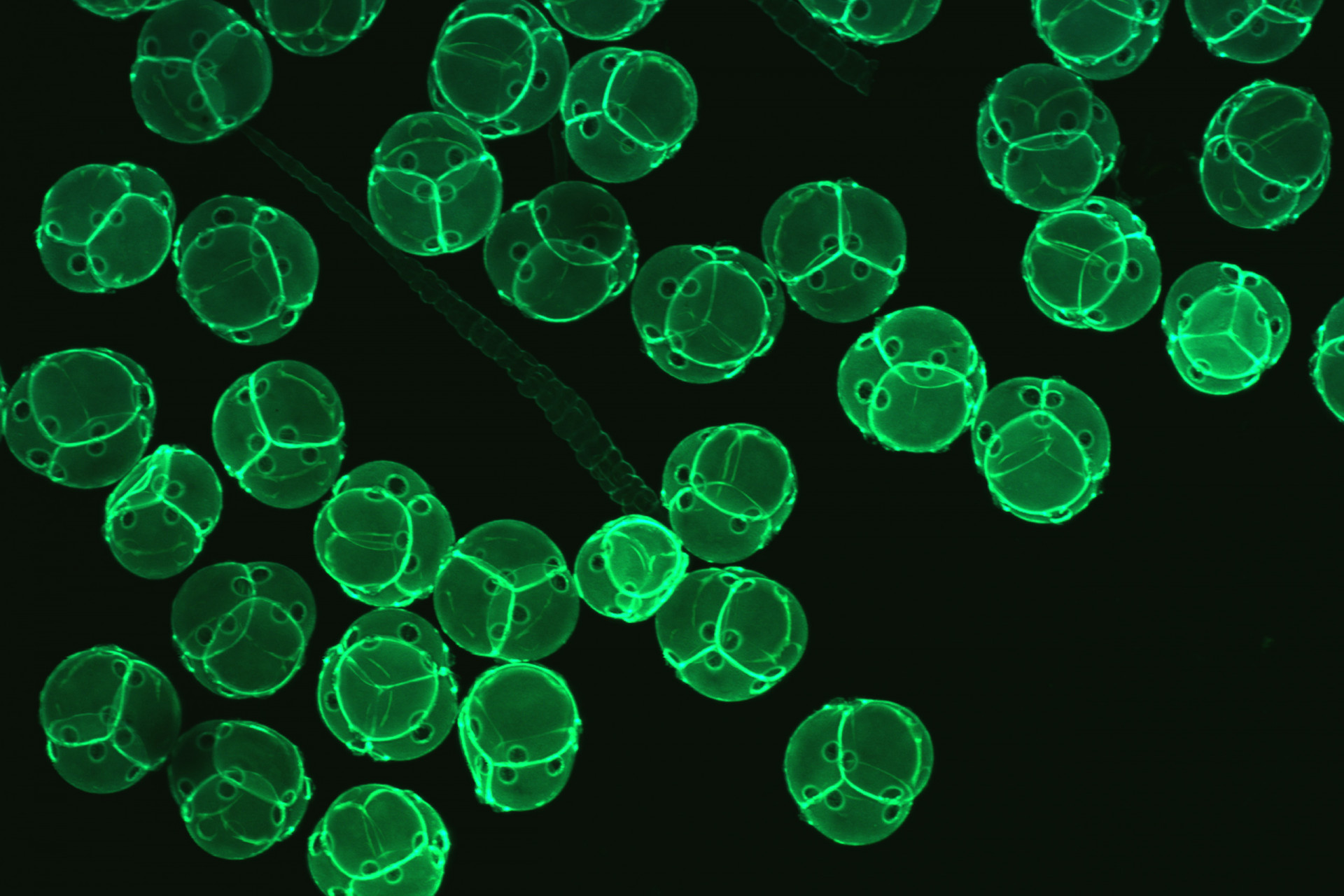
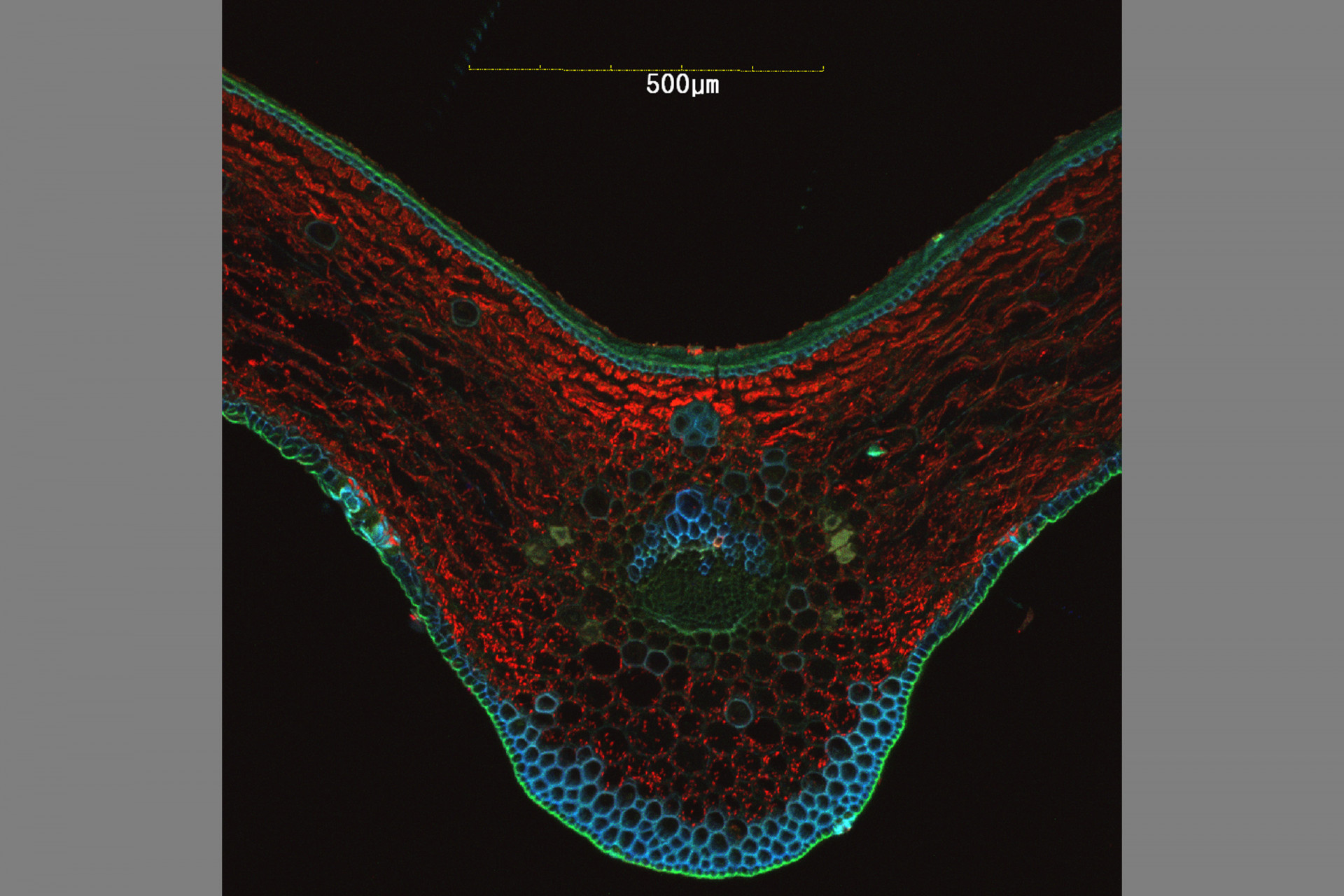
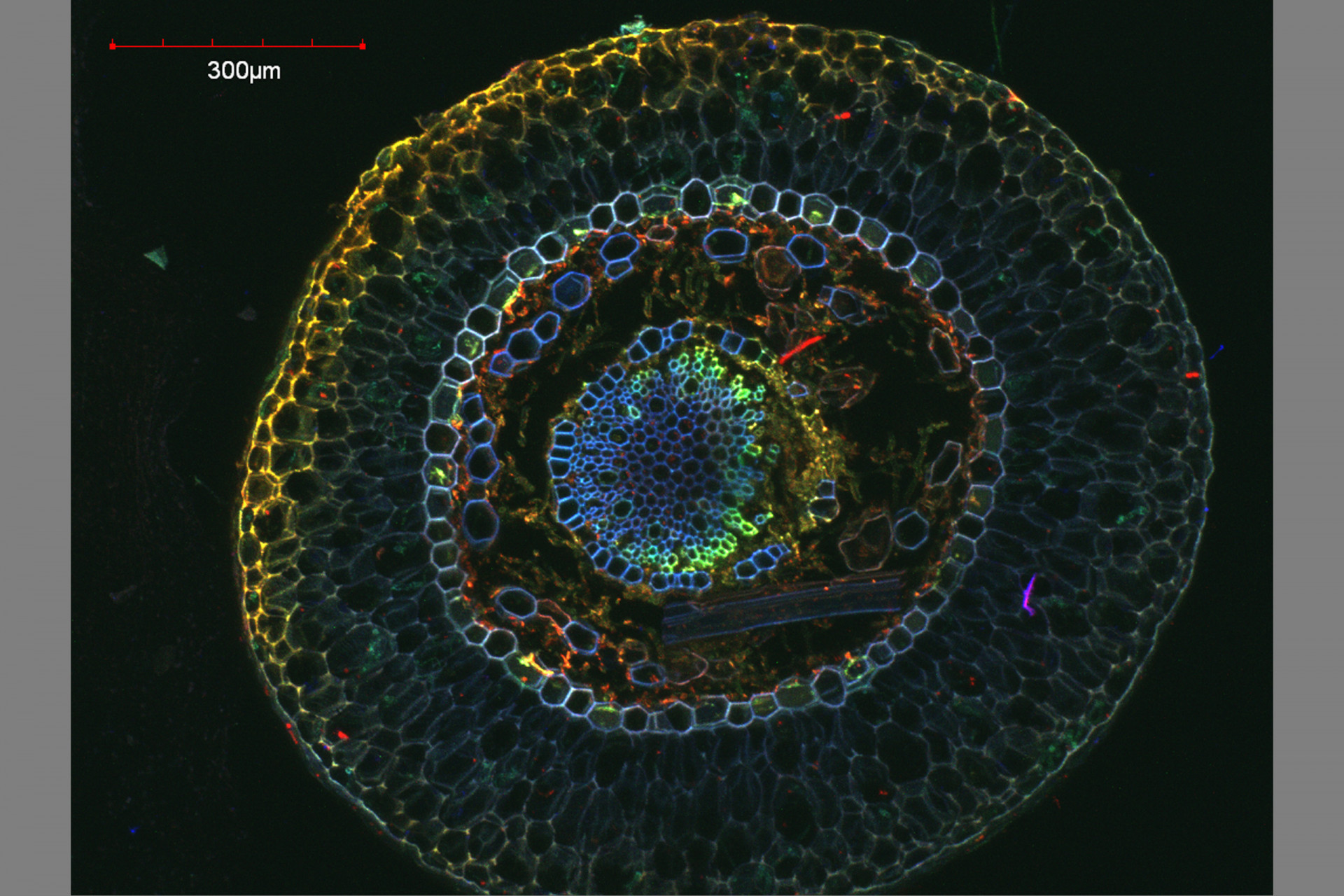
Confocal Microscopy Services
Confocal microscopy permits observation at a greater resolution and higher image quality than can be achieved using conventional optical microscopes. A laser light beam at a specific wavelength is passed over the specimen, causing excited molecules to fluoresce at a higher wavelength.
Fluorescence occurs as a result of molecules that are naturally present (autofluorescence, as in the case of chlorophyll) or due to molecules called fluorochromes, which are applied to the specimen. The large number of fluorochromes on the market are used to image different cellular structures and cause different fluorescent emissions.
The use of combinations of lasers capable of detecting and producing fluorescence at different wavelengths makes it possible to scan a specimen through a broad range of the light spectrum to visualize dyed structures at a degree of detail not possible via conventional microscopy techniques.
Because the light penetrates the specimen, the confocal microscope captures images in several different focal planes, which, when aligned via computer software, result in a three-dimensional image. The Confocal Microscopy has applications in health, biological and materials sciences.
Equipment
LSCM Olympus FV3000
Motorized Microscope Inverted IX83 (IX83P2ZF)
Integrated motorized focus module, minimum increment 0.01 μm
Objectives lens
UPLXAPO 4x NA 0.16, UPLXSAPO 10x NA 0.4, UPLXAPO 20x NA 0.8, UPLXAPO 40x NA 0.95, UPLXAPO 40xO NA 1.4 (immersion oil), UPLSAPO 40xS NA 1.4 (Silicone immersion oil), UPLSAPO 60xO NA 1.42 (immersion oil), UPLSAPO 100xS NA 1.35 (Silicone immersion oil)
Illumination:
Laser Light Violet/Visible Light Laser 405 nm: 50 mW, 488 nm: 20 mW, 561 nm: 20 mW, 640 nm: 40 mW.
The confocal microscope has 4 possible combinations of diode solid state lasers (405nm, 473nm, 559nm and 635nm) and the use of 3 observation channels plus one more for transmitted light.
Scanner Scanning Method:
2 silver-coated galvanometer scanning mirrors:
1 silver-coated resonant (High-Speed Imaging) and 1 silver-coated galvanometer scanning mirrors.
Detectors:
- High Sensitivity- Spectral Detector Cooled GaAsP photomultiplier, 2 channels
- Spectral Detector Multi-Alkali photomultiplier, 2 channels.
Fluorescence Illumination Unit
External fluorescence light source, fiber adapter to optical port of scan unit, motorized switching between LSM light path and fluorescence illumination.
Transmitted Light Detector Unit
Module with integrated external transmitted light photomultiplier detector and LED lamp, motorized switching.
General information
The Confocal and Electron Microscopy Laboratory is in the Tupper Center building 401, second floor, laboratory 223.
The service is provided in two daily sessions of 4 hours (8:00 a.m. to 12:00 md and from 1:00 p.m. to 5:00 p.m). The users will make reservations for a less two days before.
Although it is recommended that the users to bring the protocols for the development of their experiments, if necessary, the laboratory can provide basic technical support in sample preparation processes.
For more information, please contact Raineldo Urriola urriolar@si.edu or Jorge Ceballos ceballosj@si.edu.